Solve any question of Chemical Thermodynamics with-. Work and heat are the path functions.
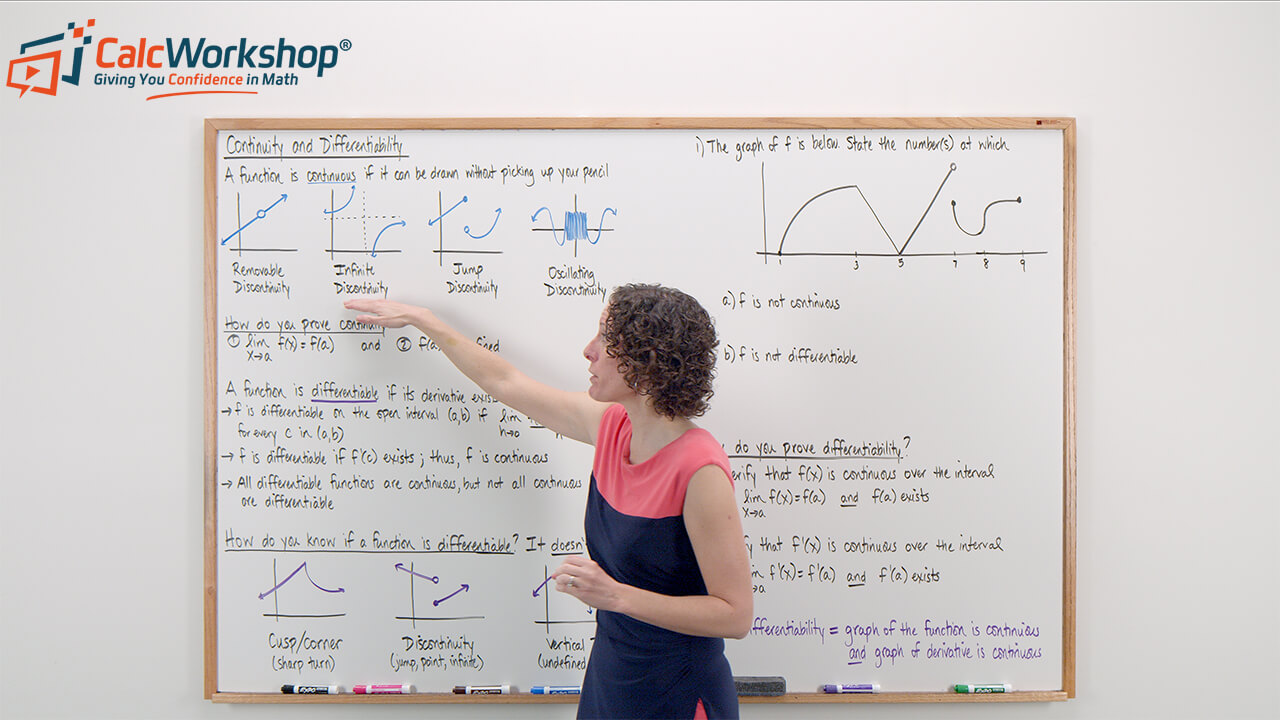
Continuity And Differentiability Fully Explained W Examples
View Notes - test4 from CHE 1220 at St.

. A internal energy B volume C work D pressure E enthalpy. They describe the system in an equilibrium state. Heat is not a state function.
You have used all of these in the ideal. Correct option is C The property whose value does not depends on path is called state function. The parameter q is not a state function because it is dependent of the path followed by the reaction.
Which of the following is not a state function. 7The real objective of Software testing is to ensure 100 defect free product. 72 States and State Functions.
Solve any question of Chemical Thermodynamics with-. Nice explanationsort of like total distance is. It is an extensive property.
Byjus Asked on June 11 2016 in Chemistry. A state function is simply one that depends only on the start and end point and not the path. Value of enthalpy internal energy and entropy depend on state and not path followed so they are state functions.
Therefore Internal energy ΔE q W. A state function is the property of the system whose value depends only on the initial and final state of the system and is independent of the path. Which of the following is a state function that can be.
I State functions are the opposite of path-dependent functions. Heat volume altitude internal energy. Macroscopic properties that a system has.
IIT JEE 2009 1 Internal energy. Heat and work are not considered state functions individually but their sum delta E is. Qw is not state function srushtisrush06 srushtisrush06 23112018 Physics Secondary School answered Which of the following are not state functions Aqw Bq Cw DU-TS 1 See answer srushtisrush06 is waiting for your help.
6Unit Testing is the highest level of testing. Quantitatively describes the pathway taken from one equilibrium state to another. 2 Reversible expansion work.
In terms of chemistry pressure volume temperature are all state functions. DeltaT T_f - T_i Moles eg. Add your answer and earn points.
Path function depends on the path followed during a process as well as the end states. Any property that depends only on the state of the system and not on the path taken to reach that state. 1 Among the following the state function s is are.
Maharashtra State Board Previous Year Question Paper With Solution for Class 12 Science. Include Q heat and W work. Which of the following is not a state function.
ΔU is the same in both scenarios since internal energy is a state function and only the final and initial conditions are relevant in calculating the value of a state function. It is a state function because it is independent of the path. If the answer is yes then it is not a state function and if the answer is no then the property is a state function.
Students upto class 102 preparing for All Government Exams CBSE Board Exam ICSE Board Exam State Board Exam JEE MainsAdvance and NEET can ask questions from any subject and get quick answers by. Work is not state function because its value depends on path followed. Which statements about state functions isare correct.
Among the followings work is not the state function as it is path dependent while all others are path independent so are state functions. Think You Can Provide A Better Answer. Ii State functions have no memory about how they arrived at a given value.
State functions or state variables depend only on the state of the system. State true or false. P_1V_1 P_2V_2 Volume eg.
AskIITians Faculty 300 Points. Maharashtra State Board Previous Year Question Paper With Solution for Class 12 Commerce. Thermodynamics definition of state function is.
State functions or state variables are those which depend only on the state of the system and not on how the state was reached. V_1T_1 V_2T_2 Temperature eg. The thermodynamic properties which depend only on initial and final states of the system and not on how the change is brought about are called the state functions.
Maharashtra State Board Previous Year Question Paper With Solution for Class 10. Iii State functions are path-dependent functions. State functions can be considered as integrals.
Major examples of state functions are internal energy entropy enthalpy free energy and Heimholtz energy. Oct 23 2015 at 1503. The function lower limit and its upper limit.
A unique platform where students can interact with teachersexpertsstudents to get solutions to their queries. Iv Heat and work are not state functions and neither is their sum a state function. Welcome to Sarthaks eConnect.
This is because integrals depend on only three things. Work and heat are major examples that are not state functions. Consider raising the temperature of 500g of water from 250C to 500C.
Maharashtra State Board Previous Year Question Paper With Solution for Class 12 Arts. 3 Irreversible expansion work. K_P K_cRTDeltan_gas Mass eg.
Heat q and work W are not state functions being path dependent. State true or false. You know plenty that you may not have identified before.
Here w represents work done and q represents the amount of heat so both of these are not state functions. Which of the following is not a state function.
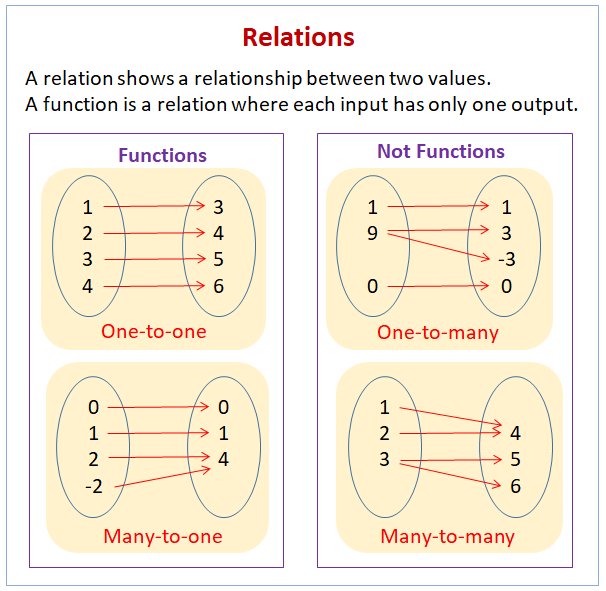
Relations And Functions Video Lessons Examples And Solutions

One To One Function Injective Function Definition Graph Examples

Thermochemistry Mcqs Pin 2 Chemistry Paper Chemistry State Function
0 Comments